COVID-19 Antigen Rapid Test Cassettes
RESULTS IN ABOUT 10 MIN
FDA Approved | Emergency Use Authorized (EUA)
For Professional Use Only

COVID-19 Antigen Rapid Test
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2 in swab specimens directly collected from individuals who are suspected of COVID-19 by their healthcare providers.
As an intended point-of-care (POC) designated test with a 10 minute processing time, CareStart™ COVID-19 Antigen Test allows effective screening of COVID-19 infection on a large scale.
-
Rapid results in 10 minutes
-
No lab equipment or additional instrument required
-
Minimally invasive Nasopharyngeal swab specimen collection
-
Detect SARS-CoV-2 nucleocapsid protein antigen
-
Lateral flow assay
Test Principles
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2 in swab specimens directly collected from symptomatic individuals who are suspected of COVID-19 by their healthcare providers.
Instructions for Use
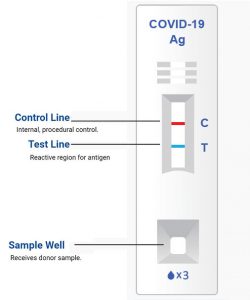
- Peel off aluminum foil seal and rotate the swab inside the extraction vial vigorously at least 5 times.
- Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Properly discard the swab.
- Close the vial by pushing the cap firmly onto the vial and mix thoroughly by flicking the bottom of the tube.
- Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well.
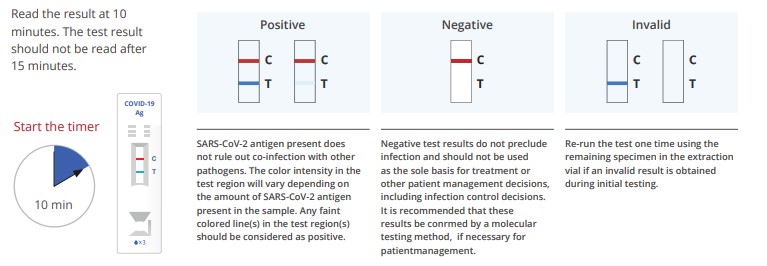
Results Interpretation
Downloads
Fact Sheets
Inserts & Brochures
Misc.
-
Lateral flow assay
-
Rapid results in 10 minutes
-
Nasopharyngeal specimen collection
-
Intended at POC setting (i.e., in patient care settings) by medical professionals with a CLIA waiver
-
Detect SARS-CoV-2 nucleocapsid protein antigen
-
Identify acute infection with 88.4% sensitivity and 100% specificity
-
Storage condition : 1-30 degrees celsius
| Manufacturer | AccessBio |
| Country of Origin | Unknown |
| Application | Rapid Test Kit |
| Contents | (20) Sealed Pouches each containing Test Device, Assay buffer, Extraction vial and cap, Specimen collection swab and
(1) of each of the following: Positive and negative control swab & |
| Number of Tests | 20 Tests/kit |
| Reading Type | Visual Read |
| Sample Type | Nasopharyngeal specimen |
| Storage Requirements | 1-30 degrees celsius |
| Test Format | Cassette Format |
| Test Method | Lateral flow assay |
| Test Name | COVID-19 Antigen |
| Test Type | Antigen Test |
| Time to Results | 10 Minute Results |
Is the Antigen Test FDA Approved?
Our Access Bio CareStart COVID-19 Rapid Antigen Test is currently FDA EUA Approved for use in point-of-care settings under a CLIA certificate of waiver. Our
What is the accuracy of the test?
Our rapid antigen test has a sensitivity (true positive rate) of 88.3% and a specificity (true negative rate) of 100%. Our point-of-care antibody test can identify positive and negative results with an accuracy of 96-97%.
Is the rapid testing process complicated and does it require specialized personnel?
No. The rapid tests we offer are intended for use in point-of-care settings. Therefore, testing processes and specimen collection are very straight-forward and can easily be administered by customer in-house medical personnel such as School Nurses or Company on-site Medical Resources.
Our Pricing
COVID-19 Antigen Rapid Test
20 tests per kit (Order Min 1 kit)
Simple Pricing
$540 per kit ($27/test)